A Solution to the Pandemic?
For anyone who hasn't looked at the news in months, we're currently in the midst of a pandemic. Fortunately, there are 5 vaccines ready for the third and final stage of clinical trials.
For anyone who hasn't looked at the news in months, we're currently in the midst of a pandemic. The virus that causes COVID-19 is part of the coronavirus family, a family of viruses that cause illnesses such as the common cold and severe acute respiratory syndrome (SARS). What makes this virus unique is that it spreads incredibly easily, partially because it's new in humans. Currently there have been at least 13 million cases of COVID in the US and over 250,000 COVID-related deaths. Many more have been impacted in other ways, with at least 20 million Americans losing their jobs. Fortunately, there is hope on the horizon, with five vaccines either in or getting ready for the third and final stage of clinical trials.
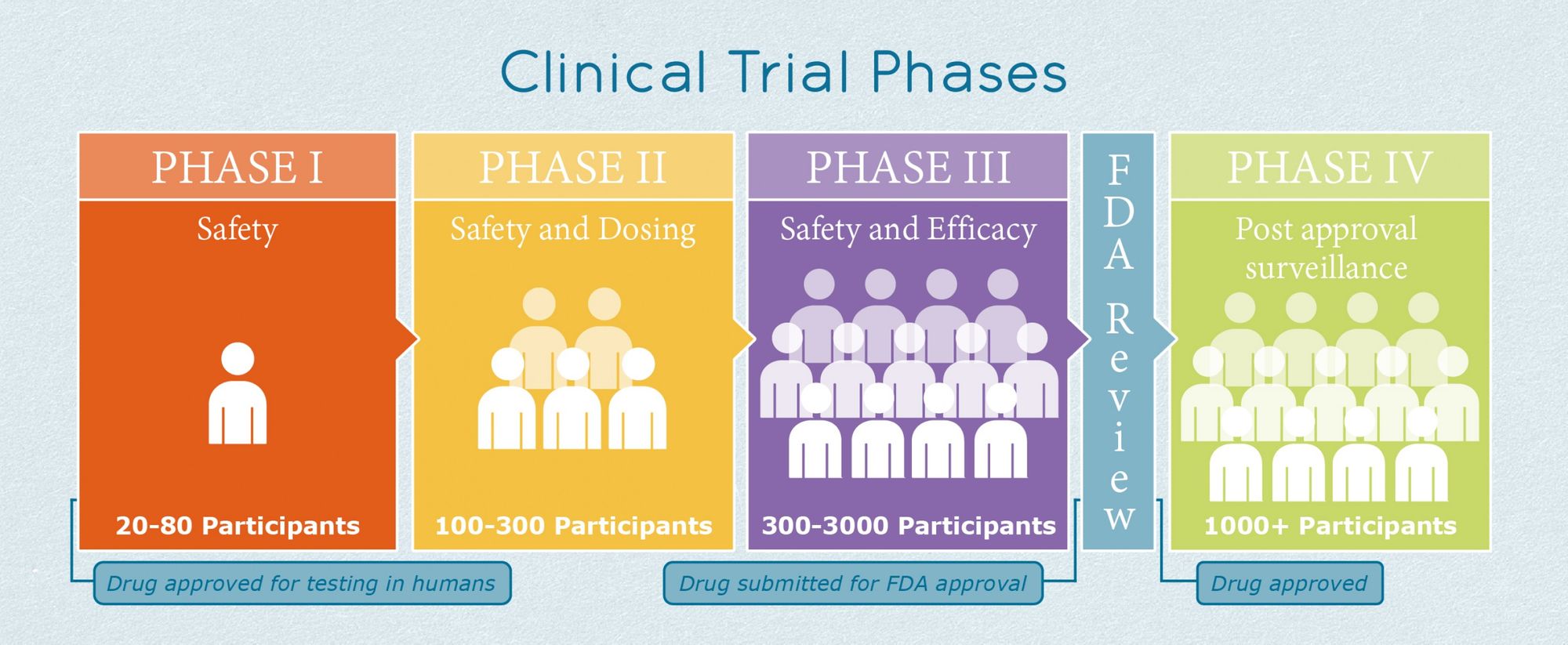
Clinical Trial Process
Creating a vaccine is only a part of the process; the vaccine must also be approved by the FDA for use. There are multiple stages involved in vaccine development: exploratory stage, pre-clinical stage, clinical development, regulatory review and approval, manufacturing, and quality control. What we'll focus on right now is the clinical development stage, which is the furthest any of the vaccines have gotten so far. The clinical development stage is when the FDA starts to get involved and the vaccine begins to be tested in humans. There are three different phases during this stage. In phase one, between 20 and 100 people are tested, primarily to see if the vaccine has any adverse side effects. During phase two, hundreds of people from different demographics are either given the vaccine or a placebo to find more safety information and study the immune response. Finally, in phase three, thousands of people are either given the vaccine or control to see how effective the vaccine is. This is the phase that the Pfizer and Morderna vaccines are currently in and as of right now, it seems like they will get FDA approval.
Pfizer Vaccine
Pfizer has developed their vaccine on their own without U.S. government funding. It was one of the first vaccines to reach phase three clinical testing. As of right now, it appears that the vaccine is over 90% effective and has no serious safety issues. If approved by the FDA, the vaccine will be made available by the spring of 2021. Pfizer believes they can produce enough vaccine doses for 25 million people by the end of 2020. However, administration of this vaccine may be a little complicated. For one, the Pfizer vaccine needs to be taken twice, 21 days apart. Most vaccines only require one dose to be effective. It also needs to be kept at temperatures below -94 F for long term storage, which means that these can only be stored in a specialized freezer. The vaccine can be kept in a refrigerator, but only if it's being stored for less than five days. However, people are optimistic about this vaccine, and the U.S. has already bought 100 million vaccinations from Pfizer.
Moderna Vaccine
Unlike Pfizer, Moderna did accept funding from the U.S. government to develop a COVID vaccine. Just like Pfizer, the Moderna vaccine is over 90% effective and will hopefully receive FDA approval by the end of the year. However, this vaccine can be stored at 25 F, which can easily be achieved by any refrigerator, and can remain in storage for 30 days. The vaccine needs to be administered twice over 28 days. Moderna believes they can have enough doses for 10 million people by the end of 2020.
How These Vaccines Got Developed So Fast
These vaccines have been developed remarkably fast; it usually takes around 10 years for a vaccine to be created and approved for use. This is partially due to new vaccine-creating technology as well as efficiencies in the process. Both the Pfizer and Moderna vaccines are mRNA vaccines, and will be the first mRNA vaccines to be approved by the U.S. Most vaccines put a weakened or dead version of the virus in our bodies to trigger an immune response, but mRNA vaccines teach cells to make a protein that will trigger an immune response. This shortens the manufacturing process because we do not need to create the weakened version of the virus, we just need to tell our body to create it.
The process in general has also been sped up. These vaccines are being created during a pandemic, the speed at which they're created and approved can stop millions of people from being infected. Because of this, companies are devoting a greater percent of their time and resources to creating these vaccines, and associations like the FDA are giving these vaccines top priority for testing. Steps that are usually done one after another are also being done at the same time right now. For example, these companies are developing vaccine manufacturing facilities before they even get approval from the FDA in order to have the vaccine ready to administer as soon as possible. Such emergency measures have allowed the progress of both vaccines.
Worries About The Vaccine
Many people are concerned with how fast these vaccines are developing and that proper precautions are not taking place. For one, the clinical trials will most likely not run long enough to find any long-term negative effects. However, most serious side effects do usually start taking place within the short term, meaning that this is not a huge concern. As of right now, the benefits of giving the vaccine a couple of months earlier far outweigh the costs of waiting to test long-term effects.
Final Thoughts
Although COVID vaccines will probably be made available by the spring of 2021, that doesn't mean we're out of this yet. Not everyone will be able to take the vaccine right away, and it will probably be given out on a priority basis. It may well be at least another year before enough people have taken the vaccine for the U.S. to have herd immunity. Regardless, these vaccines give me–and all of us–hope that better times are on their way. In the meanwhile, stay safe and wear a mask.
